
REDCap is a web-based platform that allows for the digital capture and storage of participant consent, whether participants are consented in clinic or remotely. Being able to manage consents virtually enables flexibility and a critical step to expedite the clinical research process.
New to REDCap? There are a number of resources available which can help familiarize you with the platform.
Topic-specific eConsent guides may be found below:
The REDCap eConsent Framework provides standardized tools to obtain consent and store consent documentation with a certification screen and a storage function which automatically generates a ‘hard-copy’ PDF of the signed form.

Consenting Text (i.e., what would appear in the paper consent document)

eConsent Framework (please check with your REDCap administrator if the eConsent framework does not appear in survey settings)

Create a new instrument – this will be used as your eConsent.
**If you have both a Part 1 and Part 2 consent, you will need to create two instruments.
**If you would like to use a template, you will find a standard eConsent template at the bottom of the eConsent resources page. Upload this instrument by clicking “upload instrument” on the online designer.
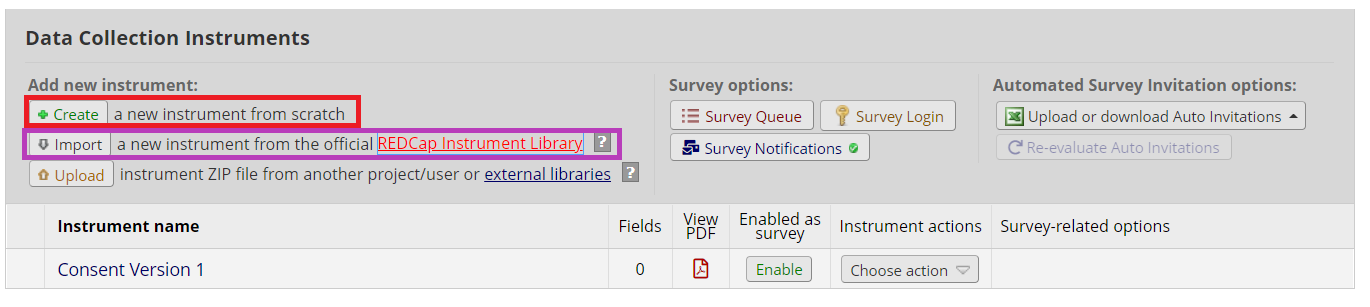
Copy your consent language into the new instrument. How many fields you use is up to you, but here are a couple of things to keep in mind:
**For especially long consents, consider using page breaks to divide up content.
**Divide sections by topic (e.g., risks, benefits, contact information). This can help to visually divide the text.
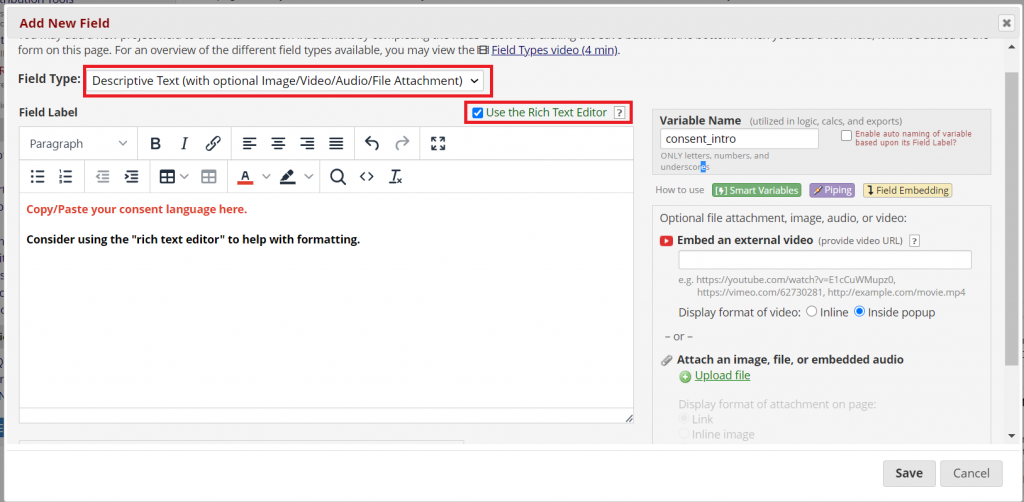
Add the signature under the consent statement and a date validated field.
Enable surveys on the “Project Setup” page.
Enable the consent instrument as a survey .
Within survey settings, scroll down to “eConsent framework”. Enable the “auto-archiver + eConsent Framework”. Once enabled, this section will expand with additional settings:
**You may include several items which will be included at the bottom of the exported PDF, including first/last name, eConsent type (e.g., pediatric). “Type” is typically used when a project contains multiple eConsent forms.
**You may also include the version number of the consent. This, along with consent tpe and participant name will appear at the bottom of the final PDF consent.
**The version of a consent form is determined by the number of revisions to the document. The first version of a consent might simply be “1” with any subsequent revisions of the consent (and thereby a new version) being “2” (and so on).
**The final section of the eConsent framework involves clearing signature fields should a participant return to the eConsent after viewing the confirmation page. After submitting an eConsent, the participant is asked to review a PDF copy of the consent and confirm that their information is correct. If the participant sees an error and returns to the document to correct or update information, they will need to re-sign. This ensures they sign the most up to date version of the document. This section is only required for studies needing Part 11 compliance, however, it may be useful for any study.